What is Uranium? How Does it Work?
- Uranium is a heavy metal which has been used as an abundant source of concentrated energy for over 60 years.
- Uranium occurs in most rocks in concentrations of 2-to-4 parts per million and is as common in the Earth's crust as tin, tungsten and molybdenum. Uranium occurs in seawater, and can be recovered from the oceans.
- Uranium was discovered in 1789 by Martin Klaproth, a German chemist, in the mineral called pitchblende. It was named after the planet Uranus, which had been discovered eight years earlier.
- Uranium was apparently formed in supernovae about 6.6 billion years ago. While it is not common in the solar system, today its slow radioactive decay provides the main source of heat inside the Earth, causing convection and continental drift.
- The high density of uranium means that it also finds uses in the keels of yachts and as counterweights for aircraft control surfaces, as well as for radiation shielding.
- Uranium has a melting point of 1132°C. The chemical symbol for uranium is U.
The uranium atom
On a scale arranged according to the increasing mass of their nuclei, uranium is one of the heaviest of all the naturally-occurring elements (hydrogen is the lightest). Uranium is 18.7 times as dense as water.
Like other elements, uranium occurs in several slightly differing forms known as 'isotopes'. These isotopes differ from each other in the number of uncharged particles (neutrons) in the nucleus. Natural uranium as found in the Earth's crust is a mixture largely of two isotopes: uranium-238 (U-238), accounting for 99.3% and uranium-235 (U-235) about 0.7%.
The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy. It is therefore said to be 'fissile' and we use the expression 'nuclear fission'.
Meanwhile, like all radioactive isotopes, they decay. U-238 decays very slowly, its half-life being about the same as the age of the Earth (4500 million years). This means that it is barely radioactive, less so than many other isotopes in rocks and sand. Nevertheless it generates 0.1 watts/tonne as decay heat and this is enough to warm the Earth's core. U-235 decays slightly faster.
Energy from the uranium atom
The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). When the nucleus of a U-235 atom captures a moving neutron it splits in two (fissions) and releases some energy in the form of heat, also two or three additional neutrons are thrown off. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, a fission 'chain reaction' can be achieved. When this happens over and over again, many millions of times, a very large amount of heat is produced from a relatively small amount of uranium.
It is this process, in effect 'burning' uranium, which occurs in a nuclear reactor. The heat is used to make steam to produce electricity.
Examples of nuclear fissioning of uranium-235
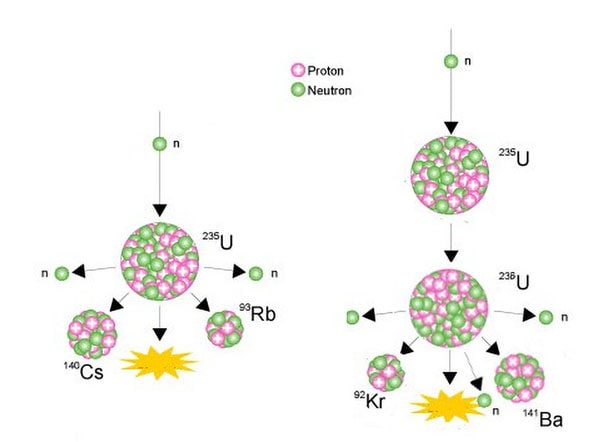
Source: World Nuclear Association
Inside the reactor
Nuclear power stations and fossil-fuelled power stations of similar capacity have many features in common. Both require heat to produce steam to drive turbines and generators. In a nuclear power station, however, the fissioning of uranium atoms replaces the burning of coal or gas. In a nuclear reactor the uranium fuel is assembled in such a way that a controlled fission chain reaction can be achieved. The heat created by splitting the U-235 atoms is then used to make steam which spins a turbine to drive a generator, producing electricity.
The chain reaction that takes place in the core of a nuclear reactor is controlled by rods which absorb neutrons and which can be inserted or withdrawn to set the reactor at the required power level.
The fuel elements are surrounded by a substance called a moderator to slow the speed of the emitted neutrons and thus enable the chain reaction to continue. Water, graphite and heavy water are used as moderators in different types of reactor.
Because of the kind of fuel used (i.e. the concentration of U-235, see below), if there is a major uncorrected malfunction in a reactor the fuel may overheat and melt, but it cannot explode like a bomb.
A typical 1000 megawatt (MWe) reactor can provide enough electricity for a modern city of up to one million people.
A Pressurized Water Reactor (PWR)
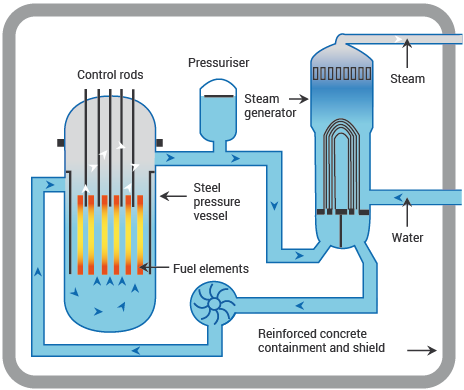
Source: World Nuclear Association
Uranium and plutonium
Whereas the U-235 nucleus is 'fissile', that of U-238 is said to be 'fertile'. This means that it can capture one of the neutrons which are flying about in the core of the reactor and become (indirectly) plutonium-239, which is fissile. Pu-239 is very much like U-235, in that it fissions when hit by a neutron and this yields a similar amount of energy.
Because there is so much U-238 in a reactor core (most of the fuel), these reactions occur frequently, and in fact about one-third of the fuel's energy yield comes from 'burning' Pu-239.
But sometimes a Pu-239 atom simply captures a neutron without splitting, and it becomes Pu-240. Because the Pu-239 is either progressively 'burned' or becomes Pu-240, the longer the fuel stays in the reactor the more Pu-240 is in it. (The significance of this is that when the spent fuel is removed after about three years, the plutonium in it is not suitable for making weapons but can be recycled as fuel.)
From uranium ore to reactor fuel
Uranium ore can be mined by underground or open-cut methods, depending on its depth. After mining, the ore is crushed and ground up. Then it is treated with acid to dissolve the uranium, which is recovered from solution.
Uranium may also be mined by in situ leaching (ISL), where it is dissolved from a porous underground ore body in situ and pumped to the surface.
The end product of the mining and milling stages, or of ISL, is uranium oxide concentrate (U3O8). This is the form in which uranium is sold.
Before it can be used in a reactor for electricity generation, however, it must undergo a series of processes to produce a useable fuel.
For most of the world's reactors, the next step in making the fuel is to convert the uranium oxide into a gas, uranium hexafluoride (UF6), which enables it to be enriched. Enrichment increases the proportion of the uranium-235 isotope from its natural level of 0.7% to 4-5%. This enables greater technical efficiency in reactor design and operation, particularly in larger reactors, and allows the use of ordinary water as a moderator.
After enrichment, the UF6 gas is converted to uranium dioxide (UO2) which is formed into fuel pellets. These fuel pellets are placed inside thin metal tubes, known as fuel rods, which are assembled in bundles to become the fuel elements or assemblies for the core of the reactor. In a typical large power reactor there might be 51,000 fuel rods with over 18 million pellets.

A worker holds up a newly made fuel pellet (Image courtesy Kazatomprom)
For reactors which use natural uranium as their fuel (and hence which require graphite or heavy water as a moderator) the U3O8 concentrate simply needs to be refined and converted directly to uranium dioxide.
When the uranium fuel has been in the reactor for about three years, the used fuel is removed, stored, and then either reprocessed or disposed of underground (see Nuclear Fuel Cycle or Radioactive Waste Management).
Who uses nuclear power?
About 9% of the world's electricity is generated from uranium in nuclear reactors. This amounts to over 2500 TWh each year, as much as from all sources of electricity worldwide in 1960.
It comes from about 440 nuclear reactors with a total output capacity of about 400,000 megawatts (MWe) operating in 31 countries. About 70 more reactors are under construction and about 110 are planned.
Armenia, Belarus, Belgium, Bulgaria, Czech Republic, Finland, France, Hungary, Slovakia, Slovenia, South Korea and Ukraine all get 30% or more of their electricity from nuclear reactors. The USA has over 90 operable reactors, supplying 20% of its electricity. France gets about 70% of its electricity from uranium.
Over the 60 years that the world has enjoyed the benefits of cleanly-generated electricity from nuclear power, there have been about 18,500 reactor-years of operational experience.
See also Nuclear Generation by Country.
Who has and who mines uranium?
Uranium is widespread in many rocks, and even in seawater. However, like other metals, it is seldom sufficiently concentrated to be economically recoverable. Where it is, we speak of an orebody. In defining what is ore, assumptions are made about the cost of mining and the market price of the metal. Uranium reserves are therefore calculated as tonnes recoverable up to a certain cost.
Uranium resources to $130/kg U by country in 2023 (reasonably assured resources plus inferred resources)
| tonnes U | percentage of world | |
| Australia |
1,671,200
|
28%
|
|---|---|---|
| Kazakhstan |
813,900
|
14%
|
| Canada |
582,000
|
10%
|
| Namibia |
497,900
|
8%
|
| Russia | 476,600 | 8% |
| Niger | 336,000 | 6% |
| South Africa | 320,900 | 5% |
| China | 270,500 | 5% |
| Brazil | 167,800 | 3% |
| Mongolia | 144,600 | 2% |
| Ukraine |
106,700
|
2%
|
| Botswana |
87,200
|
1%
|
| USA |
67,800
|
1%
|
| Tanzania | 57,700 | 1% |
| Other |
324,900
|
5%
|
| World total |
5,925,700
|
100%
|
Identified resources recoverable (reasonably assured resources plus inferred resources), to $130/kg U, 1/1/23, from OECD NEA & IAEA, Uranium 2024: Resources, Production and Demand ('Red Book'). The total recoverable identified resources to $260/kg U is 7.935 million tonnes U.
Production from mines (tonnes U)
| Country | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|---|---|---|---|---|---|
| Kazakhstan | 23,607 | 24,689 | 23,321 | 21,705 | 22,808 | 19,477 | 21,819 | 21,227 | 21,109 | 23,270 |
| Canada | 13,325 | 14,039 | 13,116 | 7001 | 6938 | 3885 | 4693 | 7351 | 11,001 | 14,309 |
| Namibia | 2993 | 3654 | 4224 | 5525 | 5476 | 5413 | 5753 | 5611 | 6986 | 7333 |
| Australia | 5654 | 6315 | 5882 | 6517 | 6613 | 6203 | 4192 | 4553 | 4693 | 4598 |
| Uzbekistan (est.) | 2385 | 3325 | 3400 | 3450 | 3500 | 3500 | 3516 | 3561 | 4000 | 4000 |
| Russia | 3055 | 3004 | 2917 | 2904 | 2911 | 2846 | 2635 | 2508 | 2710 | 2738 |
| China (est.) | 1616 | 1616 | 1692 | 1885 | 1885 | 1885 | 1600 | 1700 | 1600 | 1600 |
| Niger | 4116 | 3479 | 3449 | 2911 | 2983 | 2991 | 2248 | 2020 | 1130 | 962 |
| India (est.) | 385 | 385 | 421 | 423 | 308 | 400 | 600 | 600 | 485 | 500 |
| South Africa (est.) | 393 | 490 | 308 | 346 | 346 | 250 | 192 | 200 | 200 | 200 |
| Ukraine (est.) | 1200 | 808 | 707 | 790 | 800 | 744 | 455 | 100 | 340 | 288 |
| USA | 1256 | 1125 | 940 | 582 | 58 | 6 | 8 | 75 | 19 | 260 |
| Others | 357 | 277 | 85 | 116 | 116 | 131 | 95 | 108 | 161 | 155 |
| World total tU | 60,342 | 63,207 | 60,462 | 54,154 | 54,742 | 47,731 | 47,805 | 49,614 | 54,433 | 60,213 |
| World total tU3O8 | 71,158 | 74,536 | 71,299 | 63,861 | 64,554 | 56,286 | 46,374 | 58,507 | 64,190 | 71,006 |
| % of world demand | 98% | 96% | 93% | 80% | 81% | 74% | 76% | 76% | 83% | 90% |
Data from World Nuclear Association. NB: the figures in this table are liable to change as new data becomes available. Totals may not sum exactly due to rounding.
Mining methods have been changing. In 1990, 55% of world production came from underground mines, but this shrunk dramatically to 1999, with 33% then. From 2000 the new Canadian mines increased it again. In situ leach (ISL, also called in situ recovery, ISR) mining has been steadily increasing its share of the total, mainly due to Kazakhstan, and in 2022 accounted for over 55% of production:
| Method | Percentage of total |
| In situ leach (ISL) | 52% |
| Underground & open pit (except Olympic Dam) | 44% |
| By-product | 4% |
Other uses of nuclear energy
Uranium is sold only to countries which are signatories of the Nuclear Non-Proliferation Treaty (NPT), and which allow international inspection to verify that it is used only for peaceful purposes.
Many people, when talking about nuclear energy, have only nuclear reactors (or perhaps nuclear weapons) in mind. Few people realise the extent to which the use of radioisotopes has changed our lives over the last few decades.
Using relatively small special-purpose nuclear reactors, it is possible to make a wide range of radioactive materials (radioisotopes) at low cost. For this reason the use of artificially-produced radioisotopes has become widespread since the early 1950s, and there are now about 220 'research' reactors in 56 countries producing them. These are essentially neutron factories rather than sources of heat.
Radioisotopes
In our daily life we need food, water and good health. Today, radioactive isotopes play an important part in the technologies that provide us with all three. They are produced by bombarding small amounts of particular elements with neutrons.
In medicine, radioisotopes are widely used for diagnosis and research. Radioactive chemical tracers emit gamma radiation which provides diagnostic information about a person's anatomy and the functioning of specific organs. Radiotherapy also employs radioisotopes in the treatment of some illnesses, such as cancer. About one person in two in the Western world is likely to experience the benefits of nuclear medicine in their lifetime. More powerful gamma sources are used to sterilize syringes, bandages and other medical utensils – gamma sterilization of equipment is almost universal.
In the preservation of food, radioisotopes are used to inhibit the sprouting of root crops after harvesting, to kill parasites and pests, and to control the ripening of stored fruit and vegetables. Irradiated foodstuffs are accepted by world and national health authorities for human consumption in an increasing number of countries. They include potatoes, onions, dried and fresh fruits, grain and grain products, poultry and some fish. Some prepacked foods can also be irradiated.
In the growing of crops and breeding livestock, radioisotopes also play an important role. They are used to produce high yielding, disease-resistant and weather-resistant varieties of crops, to study how fertilizers and insecticides work, and to improve the productivity and health of domestic animals.
Industrially, and in mining, they are used to examine welds, to detect leaks, to study the rate of wear of metals, and for on-stream analysis of a wide range of minerals and fuels.
There are many other uses. A radioisotope derived from the plutonium formed in nuclear reactors is used in most household smoke detectors.
Radioisotopes are used to detect and analyse pollutants in the environment, and to study the movement of surface water in streams and also of groundwater.
See also The Many Uses of Nuclear Technology.
Other reactors
There are also other uses for nuclear reactors. About 200 small nuclear reactors power some 150 ships, mostly submarines, but ranging from icebreakers to aircraft carriers. These can stay at sea for long periods without having to make refuelling stops. In the Russian Arctic where operating conditions are beyond the capability of conventional icebreakers, very powerful nuclear-powered vessels operate year-round, where previously only two months allowed northern access each year.
The heat produced by nuclear reactors can also be used directly rather than for generating electricity. In Sweden, Russia and China, for example, surplus heat is used to heat buildings. Nuclear heat may also be used for a variety of industrial processes such as water desalination. Nuclear desalination is likely to be a major growth area in the next decade.
High-temperature heat from nuclear reactors is likely to be employed in some industrial processes in future, especially for making hydrogen.
Military sources of fuel
Both uranium and plutonium were used to make bombs before they became important for making electricity and radioisotopes. The type of uranium and plutonium for bombs is different from that in a nuclear power plant. Bomb-grade uranium is highly-enriched (>90% U-235, instead of up to 5%); bomb-grade plutonium is fairly pure Pu-239 (>90%, instead of about 60% in reactor-grade) and is made in special reactors.
Since the 1990s, due to disarmament, a lot of military uranium has become available for electricity production. The military uranium is diluted about 25:1 with depleted uranium (mostly U-238) from the enrichment process before being used in power generation. Over two decades to 2013 one-tenth of US electricity was made from Russian weapons uranium.
Related information
The Many Uses of Nuclear TechnologyNuclear Power in the World Today
Nuclear Fuel Cycle Overview
Nuclear Energy and Sustainable Development