In-Situ Leach Mining of Uranium
- In 2022, 56% of world uranium mined was from by in situ leach (ISL, also called in situ recovery, ISR) methods. Most uranium mining in the USA, Kazakhstan and Uzbekistan is now by ISL, also known as in situ recovery (ISR).
- ISL mining of uranium is undertaken in Australia, China, and Russia as well.
- In USA, ISL is seen as the most cost effective and environmentally acceptable method of mining, and other experience supports this.
Conventional mining involves removing mineralized rock (ore) from the ground, breaking it up and treating it to remove the minerals being sought.
In situ leaching (ISL), also known as solution mining, or in situ recovery (ISR) in North America, involves leaving the ore where it is in the ground, and recovering the minerals from it by dissolving them and pumping the pregnant solution to the surface where the minerals can be recovered. Consequently there is little surface disturbance and no tailings or waste rock generated. However, the orebody needs to be permeable to the liquids used, and located so that they do not contaminate groundwater away from the orebody.
Uranium ISL uses the native groundwater in the orebody which is fortified with a complexing agent and in most cases an oxidant. It is then pumped through the underground orebody to recover the minerals in it by leaching. Once the pregnant solution is returned to the surface, the uranium is recovered in much the same way as in any other uranium plant (mill).
In Australian ISL mines (Beverley, Four MIle, and Honeymoon) the oxidant used is hydrogen peroxide and the complexing agent sulfuric acid. Kazakh ISL mines generally do not employ an oxidant but use much higher acid concentrations in the circulating solutions. ISL mines in the USA use an alkali leach due to the presence of significant quantities of acid-consuming minerals such as gypsum and limestone in the host aquifers. Any more than a few percent carbonate minerals means that alkali leach must be used in preference to the more efficient acid leach. One US ISL mine – Lance – is planning to use acid leach which has proved much more effective in tests.
In 2022, a total of 27,773 tU was produced by ISL, most of this in Kazakhstan, but with 3520 tU in Uzbekistan, and lesser amounts in the USA, Australia, China and Russia. This was 56% of world total production, a share which has risen significantly from 16% in 2000.
The Australian government has published a best practice guide for ISR mining of uranium, which takes account of international differences.
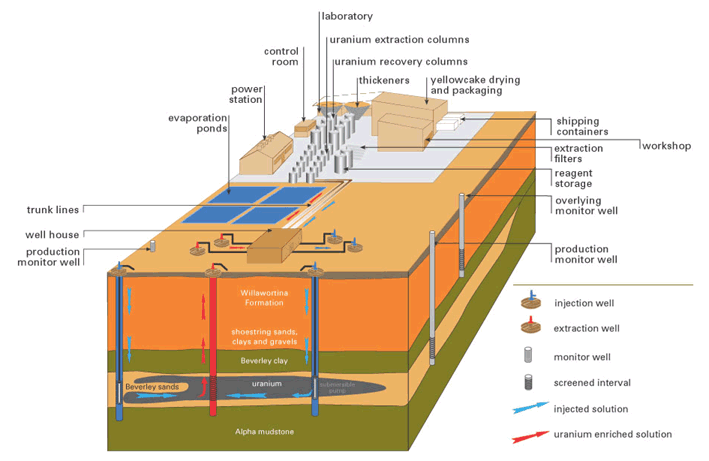
Figure 1: Pictorial representation of the ISL process (source: Heathgate Resources)
In either the acid or alkali leaching method the fortified groundwater is pumped into the aquifer via a series of injection wells where it slowly migrates through the aquifer leaching the uranium bearing host sand on its way to strategically placed extraction wells where submersible pumps pump the liquid to the surface for processing.
ISL uranium mining was first tried on an experimental basis in Wyoming during the early 1960s. The first commercial mine began operating in 1974. Today virtually all Kazakh and Uzbek, and most US uranium production comes from ISL mining. Several projects are licensed to operate there, (in Wyoming, Nebraska and Texas) and most of the operating mines date from the 1990s. They are small (under 1000 t/yr) but they supply most of the US uranium production. Russia’s Khiagda mine has ramped up to 1000 t/yr.
ISL can also be applied to other minerals such as copper and gold.
Uranium deposits suitable for ISL occur in permeable sand or sandstones, confined above and below by impermeable strata, and which are below the water table. They may either be flat, or "roll front" – in cross section, C-shaped deposits within a permeable sedimentary layer.
Such deposits were formed by the lateral movement of groundwater bearing oxidized uranium minerals through the aquifer, with precipitation of the minerals occurring when the oxygen content decreased, along extensive oxidation-reduction interfaces. The uranium minerals are usually uraninite (oxide) or coffinite (silicate) coatings on individual sand grains. See also Appendix. The ISL process essentially reverses this ore genesis, in a much shorter time frame.
There are two operating regimes for ISL, determined by the geology and groundwater. If there is significant calcium in the orebody (as limestone or gypsum, more than 2%), alkaline (carbonate) leaching must be used. Otherwise, acid (sulfate) leaching is generally better. In this case the leach solution is at a pH of 2.5-3.0, about the same as vinegar. Acid leaching gives higher uranium recovery – 70-90% – compared with 60-70% for alkaline leach, and operating costs are about half those of alkaline leach.
Techniques for ISL have evolved to the point where it is a controllable, safe, and environmentally benign method of mining which operates under strict operational and regulatory controls. Due to the low capital costs (relative to conventional mining) it can often be a more effective method of mining low-grade uranium deposits. The high-grade Phoenix ISR project in Canada will use freeze wall confinement for the leach solution, an innovation for ISL.
ISL wellfield
The design of ISL wellfields varies greatly depending on the local conditions such as permeability, sand thickness, deposit type, ore grade and distribution. Whatever the type of pattern used, there is a mixture of injection wells, to introduce the leach solution to the orebody, and extraction wells with submersible pumps used to deliver pregnant solution to the processing plant. Wells are typical of normal water bores.
Where large sheet-like deposits exist, such as in Kazakhstan, rows of injection wells interleafed with rows of extraction wells can be used cost effectively as shown in Figure 2.


Figure 2. Alternating lines of injection and extraction
This pattern has a relatively low installation cost and is simple to install. However the time taken to recover the uranium under leach is extended due to the large distances between the well types (typically 50-60m).
In most western applications (and Kazakh operations in channels narrower than 60m) closer spaced patterns are employed to recover the uranium at a faster rate (per unit area) than the alternating line patterns. The most common type of pattern employed as illustrated in Figure 3 are:
- Five spot pattern (usually 20-30 m between wells).
- Seven spot pattern (usually 13.5-20 m between wells).

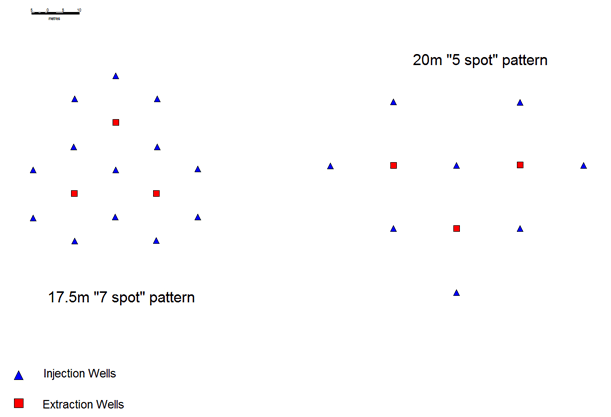
Figure 3: Five and seven spot patterns of injection and extraction
These tighter patterns are generally used effectively in narrower palaeochannel type deposits where flexibility in the installation is needed. The installed costs of these wellfields are generally higher, so to ensure maximum recovery of the uranium, the following secondary measures can be taken:
- Flow reversals – converting injection wells to extraction wells where required.
- Infill wells – to increase recovery from higher grade portions of the wellfield.


Part of Beverley wellfield, Heathgate Resources
In Australia installed wells are hydraulically pressure tested to 150% of their design operating pressure to ensure no leakage to overlying aquifers is possible. Operating wells are also re-tested after a period of 12 months of operation.
Whichever pattern type is used, the wellfields (usually a production unit that feeds to a single header house) are progressively established over the orebody as uranium is depleted. A series of monitor wells are situated around each mineralized zone to detect any movement of mining fluids outside the mining area. The wells are cased to ensure that liquors only flow to and from the ore zone and do not affect any overlying aquifers.
In the USA the production life of an individual ISL well pattern is typically one to three years. Most of the uranium is recovered during the first six months of the operation. The most successful operations have achieved a total overall recovery of about 80% of the ore, the minimum is about 60%. In Australia individual well patterns can operate from between 6 and 18 months with target recoveries of around 70% in 12 months.
The progressive flow through the aquifer also traps clay and silt in the permeable sediments. These can be dislodged to some extent by using higher pressure injection or by reversing the flow between injection and production wells. However the flow capacity of injection wells is generally always on a downward trend thought the life of the well.
Uranium recovery
The submersible pumps initially extract native groundwater from the host aquifer prior to the addition of uranium complexing reagents (acid or alkaline) and an oxidant (hydrogen peroxide or oxygen) before injection into the wellfield. The leach liquors pass through the ore to oxidize and dissolve the uranium minerals in situ.
Depending on the type of leaching environment used the uranium will be complexed as either a uranyl sulphate, predominantly UO2(SO4)34-, in acid leach conditions or a uranyl carbonate, predominantly UO2(CO3)34- in a carbonate leach system. This can then be precipitated with an alkali, e.g. as sodium or magnesium diuranate.
In either case the pregnant solution from the production wells is pumped to the treatment plant where the uranium is recovered in a resin/polymer ion exchange (IX) or liquid ion exchange (solvent extraction – SX) system.


Figure 4: Image courtesy Heathgate Resources
IX is used in the vast majority of ISL operations in Kazakhstan, the USA and Australia. In terms of operating and capital costs IX is the preferred processing option. In situations where the groundwater has a high concentration of ions that may compete with the uranyl complexes for active resin/polymer sites, such as chloride and nitrates, the use of IX becomes unattractive due to low uranium loadings on the resin/polymer. (As a general rule if chloride concentrations in the groundwater is above 5-6 g/L the capture of uranium by IX becomes uneconomical.) SX is often better with very saline groundwater (17-20 g/L) as at Honeymoon, though other process challenges can arise. Honeymoon is likely to change to IX when it reopens.
Further treatment for IX in Australia involves stripping the uranium from the resin/polymer either with a strong acid or chloride solution or a combination of both in a batch operation. In Kazakh operations the resins/polymers are generally stripped with a nitrate solution in a semi-continuous cycle. There are advantages and disadvantages with both systems and the applicability of either will again depend on the quality of the groundwater used. The pregnant solution produced by the stripping cycle is then precipitated by the addition of ammonia, hydrogen peroxide, caustic soda or caustic magnesia. Peroxide products can be dried at low temperatures to produce a product containing about 80% U3O8. However ammonium or sodium diuranate products must be dried at high temperatures to convert the product to 100% U3O8.
SX is a continuous loading/stripping cycle involving the use of an organic liquid (usually a kerosene based product) to carry the extractant which removes the uranium from solution. The uranium is then stripped from the loaded organic liquid using ammonia followed by an ammonia precipitation. The resultant slurry is then dried at high temperature as per the IX process.
After recovery of the uranium, the barren solution is re-fortified with oxidant and complexing agent before being returned to the wellfield via the injection wells. However, a small flow (about 0.5%) is bled off to maintain a pressure gradient in the wellfield and this, with some solutions from surface processing, is treated as waste. This waste water contains various dissolved ions such as chloride, sulphate, sodium, radium, arsenic and iron from the orebody and is reinjected into approved disposal wells in a depleted portion of the orebody. This bleed of process solution ensures that there is a steady flow into the wellfield from the surrounding aquifer, and serves to restrict the flow of mining solutions away from the mining area.
Acid consumption in acid leach environments is variable depending on operating philosophy and geological conditions. In general, the acid consumption in Australian ISL mines is only a fraction of that used in a Kazakh mine (per kilogram of uranium produced). A general figure for Kazakh ISL production is about 40 kg acid per kgU, though other figures of up to twice that are quoted and some mines are a bit lower. Beverley in Australia in 2007 was 7.7 kg/kgU. Unit power consumption is about 19 kWh/kgU (16 kWh/kg U3O8) in Australia and around 33 kWh/kgU in Kazakhstan.
Remote ion exchange
For very small orebodies which are amenable to ISL mining, a central process plant may be distant from the mine, so a satellite plant will be set up. This does no more than provide a facility to load the ion exchange (IX) resin/polymer so that it can be trucked to the central plant in a bulk trailer for stripping. Hence very small deposits can become viable, since apart from the wellfield, little capital expenditure is required at the mine site.
Remote ion exchange is being used in Wyoming and Texas in the USA, in the former as toll milling. It is used for Four Mile in South Australia, where for historical reasons the main treatment plant at Beverley is several kilometres distant.


Beverley plant, Heathgate Resources
ISL in Kazakhstan
In 2010 there were 19 ISL mines operating in Kazakhstan, making it by far the world leader in using ISL methods. Initial tests using ISL commenced in 1970 and were successful. Kazakhstan's Reasonably Assured Resources plus Inferred Resources to US$ 130/kgU were 651,000 tU at 2009, almost all amenable to ISL extraction.
All except one of the operating and planned ISL mine groups are in the Chu-Sarysu province in the central south of the country and controlled by the state corporation Kazatomprom. Mines in the Stepnoye area have been operating since 1978, some in the Tsentralnoye area since 1982 – both in the Chu-Sarysu basin/district, which has more than half the country's known resources. Mines in the Western (No.6) area of the Syrdarya basin/district have operated since 1985. All have substantial resources. Mining is at depths of 100-300 metres, though some orebodies extend to 800 metres.
Tortkuduk, Budenovskoye, Inkai, South Inkai and Moinkum are the largest ISL mines, and Cameco's description of Inkai's operation is: Uranium occurs in sandstone aquifers as coatings on the sand grains at a depth of up to 300 metres. Uranium is largely insoluble in the native groundwater which is not potable due to naturally high concentrations of radionuclides and dissolved solids. Using a grid of injection and production wells, a mining solution containing an oxidant (sulfuric acid) is circulated through the orebody to dissolve the uranium. The uranium-bearing solution (generally containing less than 0. 1% uranium) is then pumped to a surface processing facility where the uranium is removed using ion exchange resin/polymer. The water is re-oxidized and re-injected into the orebody. The uranium is stripped from the resin/polymer, precipitated with hydrogen peroxide and then dried to form the final product, U3O8. This process is repeated to remove as much uranium as is economically feasible. When mining at the site is complete, the groundwater will be restored to its original quality.
This is a closed loop recirculation system since the water from the production well is reintroduced in the injection wells. Slightly less water is injected than is pumped to the surface to ensure that fluids are confined to the ore zones intended for extraction. Monitor wells are installed above, below and around the target zones to check that mining fluids do not move outside a permitted mining area.
ISL uranium production in Kazakhstan requires large quantities of sulfuric acid , due to relatively high levels of carbonate in the orebodies. This was a serious constraint on production over 2007-10. In 2009 Kazatomprom with other mining companies and two acid producers, KazZinc JSC and Kazakhmys, set up a coordinating council to regulate acid supplies and infrastructure. Since then acid supply has been adequate, and new acid plant capacity has been built.
See also paper Uranium and Nuclear Power in Kazakhstan.
Groundwater remediation
At established operations overseas, after ISL mining is completed, the quality of the remaining groundwater must be restored to a baseline standard determined before the start of the operation, so that any prior use can be resumed. Contaminated water drawn from the aquifer is either evaporated or treated before reinjection.
In contrast to the main US operations, the water quality at the Australian sites is very poor to start with, and it is quite unusable. Also the mine sites are remote. At Beverley the groundwater in the orebody is fairly saline – over 12 g/L total dissolved solids (TDS) and orders of magnitude too high in radionuclides for any permitted use. It flows at only about 5 metres per year. At Honeymoon the water is even more saline, and high in sulfates and radium. When oxygen input and leaching is discontinued, the water quality soon reverts to its original condition. Post mining monitoring is for three years minimum, on pH, sulfates and uranium.
At Beverley natural attenuation is monitored in line with the Geoscience Australia best practice guide 2010. Early verification of the process can be undertaken before mining ceases. Beverley North and Four Mile are similar in natural attenuation.
In Kazakhstan a test of this has been conducted at the Irkol deposit with four main parameters measured over 1985 to 1997. In four years the ISL-affected area had reduced by half, and after 12 years it was fully restored naturally.
Where active attenuation of groundwater is required, this may be by simple reagent amendment or surface treatment (IX or RO) and reinjection.
In the USA legislation requires that the water quality in the affected aquifer be restored so as to enable its pre-mining use. Usually this is potable water or stock water (usually less than 500 ppm total dissolved solids), and while not all chemical characteristics can be returned to those pre-mining, the water must be usable for the same purposes as before. Often it needs to be treated by reverse osmosis (RO), giving rise to a problem in disposing of the concentrated brine stream from this.
The new plant for the Lance project in Wyoming incorporates a restoration circuit with IX then RO to restore water quality of barren liquor to pre-mining levels.
Environment and health
Upon decommissioning, wells are sealed or capped, process facilities removed, any evaporation pond revegetated, and the land can readily revert to its previous uses.
The usual radiation safeguards are applied at an ISL mining operation, despite the fact that most of the orebody's radioactivity remains well underground and there is hence minimal increase in radon release and no ore dust. Employees are monitored for alpha radiation contamination and personal dosimeters are worn to measure exposure to gamma radiation. Routine monitoring of air, dust and surface contamination are undertaken.
Appendix
Deposits that can be mined with ISL
Sandstone-hosted uranium deposits account for approximately 18% of world uranium resources and 7% of Australia's total uranium reserves and resources. Seven sandstone-hosted uranium deposits exist within the Curnamona Province, South Australia. The largest deposits within this region are the Beverley Four Mile Deposit (Quasar Resources Pty Ltd and Alliance Resources Ltd), the Beverley Deposit (Heathgate Resources Pty Ltd) and the Honeymoon/East Kalkaroo Deposits (Uranium One). The latter two deposits are currently being mined or are permitted to be mined by in-situ leach (ISL) mining methods.
Western Australian sandstone deposits include Manyingee (Paladin Resources Ltd), Oobagooma (Paladin Resources Ltd) and, in part, Mulga Rock (Eaglefield Holdings Pty Ltd). The Angela and Pamela Deposits comprise the most well-known sandstone deposits in the Northern Territory. Large areas of low-grade uranium mineralization also occur in the Eucla Basin, South Australia. These include Warrior (Stellar Resources Ltd), Yaninee (Adelaide Resources Ltd) and the Yarranna group of deposits (Iluka Resources Ltd). These deposits are yet to be developed however some may be amenable to ISL mining methods depending on local geological, hydro-geological and economic factors.
Sandstone deposits either occur as extensive sheet-like bodies (Colorado Plateau, South Kazakhstan) or within fossil river systems called palaeochannels (Curnamona Province). Sandstone Deposits (particularly palaeochannel deposits) are usually less than 20,000 tonnes U3O8, some sheet-like sandstone deposits such as Cameco's Inkai Deposit can be large with Inkai's proven and probable reserves in excess of 80,000 tonnes U3O8. Average grades of sandstone-hosted deposits range between 0.05% to 0.40% U3O8.
In almost all cases the formation of sandstone-hosted uranium deposits occurs when uranium, transported in oxygen-rich groundwater, interacts with a reduced host rock. During this interaction the soluble hexavalant uranium (U6+) ion is converted to the insoluble tetravalent (U4+) ion which, in turn, bonds with Si, O and H to form coffinite and other uranium species. The resulting mineralization is fine-grained (often less than 20 microns) and comprises reduced uranium species; readily soluble uraninite [UO2] and coffinite [U(SiO4)0.5(OH)2] are the most common. Secondary uranium minerals such as carnotite [K2(UO2)2 (VO4)2.H2O] can also precipitate when vanadium is present, though this does not form by redox reactions, rather it precipitates in an oxidising environment as a complex U6+ mineral (with vanadium) in calcrete deposits.
Calcrete accumulations may be up to 100 km long and 5 km wide and are aquifers. ‘Valley’ calcretes in arid areas indicate an environment functioning as a giant concentrating system in which components are leached from the weathered rock of a large catchment area and the products are deposited in a relatively small well-defined area. In Australia’s northern Yilgarn catchments with granitic rocks containing 2–25 ppm U, oxidising conditions have prevailed in places to depths of 300 m, and uranium has been mobilized as U6+ complexes and transported laterally by groundwater. Where these groundwaters reach valley axes the water table rises to within 5m of the surface. There, evaporation and loss of carbon dioxide promotes precipitation, particularly of carbonates of calcium and magnesium. Where the solubility product of the concentration of ion species of uranium, vanadium and potassium exceeds the solubility product of carnotite, this mineral is precipitated in fissures or between carbonate and clay particles.
(Much of the information in this Appendix is from McKay & Miezitis, 2001, Geoscience Australia)
Notes & references
General sources
Heathgate Resources, 1998, Beverley Uranium Mine Environmental Impact Statement.
Dobrzinski, I, 1997, Beverley and Honeymoon Deposits, MESA Journal 5, April 1997.
Szymanski, W N. 1993, Energy Information Administration, Uranium Industry Annual.
Ackland, M.C. et al, 1999, The future of solution mining, ANA Conference paper.
Hunter, T, 2001, Developments in Uranium Solution Mining in Australia, ANA Conference paper.
Geoscience Australia, 2010, Australia's in situ recovery uranium mining best practice guide.
McKay, A., and Miezitis, Y. (2001) Australia’s Uranium Resources, Geology And Development Of Deposits, Geoscience Australia, ISBN 0 642 46716 1
Boystov, A, 2014, Worldwide ISL Uranium Mining Outlook, URAM 2014, IAEA Vienna
IAEA 2016, In Situ Leach Mining of Uranium: An Overview of Operations, IAEA Nuclear Energy series NF-T-1.4
Related information
AustraliaOccupational Safety in Uranium Mining
Kazakhstan
World Uranium Mining Production