Uranium Mining Overview
- In the last 60 years uranium has become one of the world’s most important energy minerals.
- It is mined and concentrated similarly to many other metals.
While uranium is used almost entirely for making electricity, a small proportion is used for the important task of producing medical isotopes. Some is also used in marine propulsion, especially naval.
Uranium is a naturally occurring element with an average concentration of 2.8 parts per million in the Earth's crust. Traces of it occur almost everywhere. It is more abundant than gold, silver or mercury, about the same as tin and slightly less abundant than cobalt, lead or molybdenum. Vast amounts of uranium also occur in the world's oceans, but in very low concentrations.
The largest-producing uranium mines in 2024
| Mine | Country | Main owner | Type | Production (tonnes U) | % of world |
|---|---|---|---|---|---|
| McCarthur River/Key Lake | Canada | Cameco | underground | 7808 | 13 |
| Cigar Lake | Canada | Cameco/Orano | underground | 6501 | 11 |
| Husab | Namibia | Swakop Uranium (CGN) | open pit | 4437 | 7 |
| Karatau (Budenovskoye 2) | Kazakhstan | Uranium One/Kazatomprom | ISL | 3299 | 6 |
| Inkai, sites 1-3 | Kazakhstan | Kazatomprom/Cameco | ISL | 2992 | 5 |
| Akdala & South Inkai 4 | Kazakhstan | Uranium One/Kazatomprom | ISL | 2803 | 5 |
| Olympic Dam | Australia | BHP Billiton | by-product/underground | 2693 | 5 |
| Moinkum & Tortkuduk | Kazakhstan | Orano/Kazatomprom | ISL | 2388 | 4 |
| Rössing | Namibia | CNNC | open pit | 2205 | 4 |
| Khorassan 1 | Kazakhstan | Kazatomprom/Uranium One | ISL | 2030 | 3 |
| Top 10 total | 37,156 | 62% |
Most of the uranium ore deposits at present supporting these mines have average grades in excess of 0.10% of uranium – that is, greater than 1000 parts per million. In the first phase of uranium mining to the 1960s, this would have been seen as a respectable grade, but today some Canadian mines have huge amounts of ore up to 20% U average grade. Other mines however can operate successfully with very low grade ores, down to about 0.02% U. Uranium mines operate in some 20 countries, though in 2024 over 60% of world production came from just 10 mines in four countries.
Some uranium is also recovered as a by-product with copper, as at Olympic Dam mine in Australia, or as by-product from the treatment of other ores, such as the gold-bearing ores of South Africa, or from phosphate deposits such as Morocco and Florida. In these cases the concentration of uranium may be as low as a tenth of that in orebodies mined primarily for their uranium content. An orebody is defined as a mineral deposit from which the mineral may be recovered at a cost that is economically viable given the current market conditions. Where a deposit holds a significant concentration of two or more valuable minerals then the cost of recovering each individual mineral is reduced as certain mining and treatment requirements can be shared. In this case, lower concentrations of uranium than usual can be recovered at a competitive cost.
Generally speaking, uranium mining is no different from other kinds of mining unless the ore is very high grade. In this case special mining techniques such as dust suppression, and in extreme cases remote handling techniques, are employed to limit worker radiation exposure and to ensure the safety of the environment and general public.
Searching for uranium is in some ways easier than for other mineral resources because the radiation signature of uranium's decay products allows deposits to be identified and mapped from the air.
Different kinds of mines
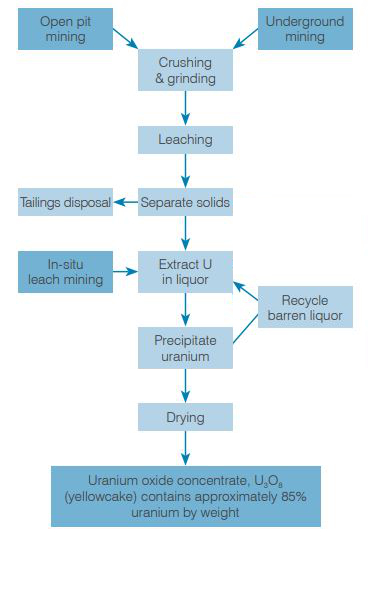
Open pit and underground mining
Where orebodies lie close to the surface, they are usually accessed by open cut mining, involving a large pit and the removal of much overburden (overlying rock) as well as a lot of waste rock. Where orebodies are deeper, underground mining is usually employed, involving construction of access shafts and tunnels but with less waste rock removed and less environmental impact. In either case, grade control is usually achieved by measuring radioactivity as a surrogate for uranium concentration.* (The radiometric device detects associated radioactive minerals which are decay products of the uranium, rather than the uranium itself.)
* About 95% of the radioactivity in the ore is from the U-238 decay series, totalling about 150 kBq/kg in ore with 0.1% U3O8. The U-238 series has 14 radioactive isotopes in secular equilibrium, thus each represents about 11 kBq/kg (irrespective of the mass proportion). When the ore is processed, the U-238 and the very much smaller masses of U-234 (and U-235) are removed. The balance becomes tailings, and at this point has about 85% of its original intrinsic radioactivity. However, with the removal of most U-238, the following two short-lived decay products in the uranium decay series (Th-234 and Pa-234) soon disappear, leaving the tailings with a little over 70% of the radioactivity of the original ore after several months. The controlling long-lived isotope then becomes Th-230 which decays with a half-life of 77,000 years to radium-226 followed by radon-222. (Supervising Scientist Group, Australia).
At Rössing in Namibia, and most of Canada's Northern Saskatchewan mines through to McClean Lake, the orebodies have been accessed by open cut mining. Other mines such as Olympic Dam in Australia, McArthur River, Rabbit Lake and Cigar Lake in Northern Saskatchewan, and Akouta in Niger are underground, up to 600 metres deep. At McClean Lake and Ranger, mining will be completed underground.
In situ leach (ISL) mining
Some orebodies lie in groundwater in porous unconsolidated material (such as gravel or sand) and may be accessed simply by dissolving the uranium and pumping it out – this is in situ leach (ISL) mining (also known in North America as in situ recovery - ISR). It can be applied where the orebody's aquifer is confined vertically and ideally horizontally. Certainly it is not licensed where potable water supplies may be threatened. Where appropriate it is certainly the mining method with least environmental impact.
ISL mining means that removal of the uranium minerals is accomplished without any major ground disturbance. Weakly acidified groundwater (or alkaline groundwater where the ground contains a lot of limestone such as in the USA) with a lot of oxygen in it is circulated through an enclosed underground aquifer which holds the uranium ore in loose sands. The leaching solution dissolves the uranium before being pumped to the surface treatment plant where the uranium is recovered as a precipitate. Most US and Kazakh uranium production is by this method.
In Australian ISL mines the oxidant used is hydrogen peroxide and the complexing agent sulfuric acid to give a uranyl sulphate. Kazakh ISL mines generally do not employ an oxidant but use much higher acid concentrations in the circulating solutions. ISL mines in the USA use an alkali leach to give a uranyl carbonate due to the presence of significant quantities of acid-consuming minerals such as gypsum and limestone in the host aquifers. Any more than a few percent carbonate minerals means that alkali leach must be used in preference to the more efficient acid leach, though the cost is often double.
In either the acid or alkali leaching method the fortified groundwater is pumped into the aquifer via a series of injection wells where it slowly migrates through the aquifer leaching the uranium bearing host sand on its way to strategically placed extraction wells where submersible pumps pump the liquid to the surface for processing.
For very small orebodies which are amenable to ISL mining, a central process plant may be distant from them so a satellite plant will be set up. This does no more than provide a facility to load the ion exchange (IX) resin/polymer so that it can be trucked to the central plant in a bulk trailer for stripping. Hence very small deposits can become viable, since apart from the wellfield, little capital expenditure is required at the mine and remote IX site.
Heap leaching
Some ore, usually very low-grade (below 0.1%U), is treated by heap leaching. Here the broken ore is stacked about 5 to 30 metres high on an impermeable pad and irrigated with acid (or sometimes alkaline) solution over many weeks. The pregnant liquor from this is collected and treated to recover the uranium, as with ISL, usually using ion exchange. After the material ceases to yield significant further uranium, it is removed and replaced with fresh ore. Recoveries are typically 50-80%. The depleted material has the potential to cause pollution so must be emplaced securely so as not to affect surface water or groundwater. Usually this will be in mined-out pits.
Milling and processing
Conventional mines have a mill where the ore is crushed and ground to liberate the mineral particles, then leached in tanks with sulfuric acid to dissolve the uranium oxides. The solution is then processed to recover the uranium. With some South African uranium recovery from gold tailings, a pressure leach is necessary.
Sometimes a physical beneficiation process is used to concentrate the ore and increase the head grade before chemical treatment. This may be radiometric sorting as at Ranger, screening/gravity, or a new process called ablation.
Most of the ore is barren rock or other minerals which remain undissolved in the leaching process. These solids or 'tailings' are separated from the uranium-rich solution, usually by allowing them to settle out. The remaining solution is filtered and the uranium is recovered in some form of ion exchange (IX) or solvent extraction (SX) system. The pregnant liquor from ISL or heap leaching is treated similarly. The uranium is then stripped from this and precipitated – see box. The final chemical precipitate is filtered and dried.
Mill chemistry
The crushed and ground ore, or the underground ore in the case of ISL mining, is leached with sulfuric acid:
UO3 + 2H+ ====> UO22+ + H2O
UO22+ + 3SO42- ====> UO2(SO4)34-
The UO2 is oxidized to UO3.
With some ores, carbonate leaching is used to form a soluble uranyl tricarbonate ion: UO2(CO3)34-. This can then be precipitated with an alkali, e.g. as sodium or magnesium diuranate.
The uranium in solution is recovered in a resin/polymer ion exchange (IX) or liquid ion exchange (solvent extraction – SX) system. The pregnant liquor from acid ISL or heap leaching is treated similarly.
Further treatment for IX involves stripping the uranium from the resin/polymer either with a strong acid or chloride solution or with a nitrate solution in a semi-continuous cycle. The pregnant solution produced by the stripping cycle is then precipitated by the addition of ammonia, hydrogen peroxide, caustic soda or caustic magnesia. Solvent extraction is a continuous loading/stripping cycle involving the use of an organic liquid to carry the extractant which removes the uranium from solution.
Typically, in solvent extraction, tertiary amines* are used in a kerosene diluent, and the phases move countercurrently.
2R3N + H2SO4 ====> (R3NH)2SO4
2 (R3NH)2SO4 + UO2(SO4)34- ====> (R3NH)4UO2(SO4)3 + 2SO42-
* "R" is an alkyl (hydrocarbon) grouping, with single covalent bond.
The loaded solvents may then be treated to remove impurities. First, cations are removed at pH 1.5 using sulfuric acid and then anions are dealt with using gaseous ammonia.
The solvents are then stripped in a countercurrent process using ammonium sulfate solution.
(R3NH)4UO2(SO4)3 + 2(NH4)2SO4 ====> 4R3N + (NH4)4UO2(SO4)3 + 2H2SO4
Precipitation of ammonium diuranate is achieved by adding gaseous ammonia to neutralize the solution (though in earlier operations caustic soda and magnesia were used).
2NH3 + 2UO2(SO4)34- ====> (NH4)2U2O7 + 4SO42-
The diuranate is then dewatered and roasted to yield U3O8 product, which is the form in which uranium is marketed and exported.
Peroxide products can be dried at ambient temperatures to produce a product containing about 80% U3O8. Ammonium or sodium diuranate products are dried at high temperatures to convert the product to uranium oxide concentrate – U3O8 – about 85% uranium by mass. This is sometimes referred to as yellowcake, though it is usually khaki.
In the case of carbonate leaching the uranyl carbonate can be precipitated with an alkali, e.g. as sodium or magnesium diuranate.
The product is then packed into 200 litre steel drums which are sealed for shipment. The U3O8 is only mildly radioactive (the radiation level one metre from a drum of freshly-processed U3O8 is about half that – from cosmic rays – on a commercial jet flight). In ISL mills the process of uranium recovery is very similar, without the need for crushing and grinding.
Tailings management and mine rehabilitation
From open cut mining, there are substantial volumes of barren rock and overburden waste. These are placed near the pit and either used in rehabilitation or shaped and revegetated where they are.
Uranium minerals are always associated with more radioactive elements such as radium and radon in the ore which arise from the radioactive decay of uranium over a few million of years. Therefore, although uranium itself is barely radioactive, the ore which is mined, especially if it is very high-grade such as in some Canadian mines, is handled with some care, for occupational health and safety reasons.
Mining methods, tailings and run-off management and land rehabilitation are subject to Government regulation and inspection. For instance in Australia the Code of Practice and Safety Guide: Radiation Protection and Radioactive Waste Management in Mining and Mineral Processing was published in 2005, and updated in 2015.
Solid waste products from the milling operation are tailings, ranging in character from slimes to coarse sands. They comprise most of the original ore and they contain most of the radioactivity in it. In particular they contain all the radium present in the original ore. At an underground mine they may be first cycloned to separate the coarse fraction which is returned underground and used for underground fill. The balance is pumped as a slurry to a tailings dam, which may be a worked-out pit as at Ranger and McClean Lake, or an engineered structure.
When radium undergoes natural radioactive decay one of the products is radon gas. Because radon and its decay products (daughters) are radioactive and because the ground rock comprising the tailings is now on the surface, measures are taken to minimize the emission of radon gas. During the operational life of a mine the material in the tailings dam is often kept covered by water to reduce surface radioactivity and radon emission (though with lower-grade ores neither pose a hazard at these levels). This water needs to be recycled or evaporated since it contains radium, which is relatively soluble. Most Australian mines and many others adopt a 'zero discharge' policy for any pollutants.
On completion of the mining operation, it is normal for the tailings dam to be covered by some two metres of clay and topsoil with enough rock to resist erosion. This is to reduce both gamma radiation levels and radon emanation rates to levels near those normally experienced in the region of the orebody, and for a vegetation cover to be established. At Ranger and Jabiluka in North Australia, tailings will finally be returned to the mine pit or underground, as was done at the now-rehabilitated Nabarlek mine. In Canada, ore treatment is often remote from the mine that the new ore comes from, and tailings are emplaced in mined-out pits wherever possible, and engineered dams otherwise.
At established ISL operations, after mining is completed the quality of the remaining groundwater must be restored to a baseline standard determined before the start of the operation so that any prior uses may be resumed. Usually this is potable water or stock water (usually less than 500 ppm total dissolved solids). Contaminated water drawn from the aquifer is either evaporated or treated before reinjection.
In contrast to the main US operations, the water quality at the Australian sites is very poor to start with, and it is quite unusable. At Beverley the original groundwater in the orebody is fairly saline and orders of magnitude too high in radionuclides for any permitted use. At Honeymoon the original water is even more saline, and high in sulfates and radium. When oxygen input and leaching are discontinued, the water quality reverts to its original condition over time.
Upon decommissioning, ISL wells are sealed or capped, process facilities removed, any evaporation pond revegetated, and the land can readily revert to its previous uses.
Mining is generally considered a temporary land use, and upon completion the area with any waste rock, overburden, and covered tailings needs to be left fit for other uses, or its original use. In many parts of the world governments hold bonds to ensure proper rehabilitation in the event of corporate insolvency.
The health of workers
In Australia all uranium mining and milling operations are undertaken under the Code of Practice and Safety Guide for Radiation Protection and Radioactive Waste Management in Mining and Mineral Processing. This was drawn up by the national government in line with recommendations of the International Commission on Radiological Protection (ICRP), but it is administered by state health and mines departments. The Code, which was updated in 1995, 2005 and again in 2015, sets strict health standards for radiation and radon gas exposure, for both workers and members of the public.
In Canada the Canadian Nuclear Safety Commission is responsible for regulating uranium mining as well as other aspects of the nuclear fuel cycle. In Saskatchewan, provincial regulations also apply concurrently, and set strict health standards for both miners and local people.
Uranium itself is only slightly radioactive. However, radon, a radioactive inert gas, is released to the atmosphere in very small quantities when the ore is mined and crushed. Radon occurs naturally in most rocks – minute traces of it are present in the air which we all breathe and it is a significant contributor to the natural radiation dose that we all receive. Because it is airborne, special care must be taken to ensure that mine worker exposure, especially in poorly ventilated mines, is limited.
Open cut mines are naturally well ventilated. The Olympic Dam and Canadian (as well as other) underground mines are ventilated with powerful fans. Radon levels are kept at a very low and certainly safe level in uranium mines. (Radon even in non-uranium mines also may need control by ventilation.)
Gamma radiation may also be a hazard to those working close to high-grade ores. It comes principally from uranium decay products in the ore, so exposure to this is regulated as required. In particular, dust is suppressed, since this represents the main potential exposure to alpha radiation as well as a gamma radiation hazard.
At the concentrations associated with uranium (and some mineral sands) mining, radioactivity is a potential health hazard. Precautions taken during the mining and milling of uranium ores to protect the health of the workers include:
- Good forced ventilation systems in underground mines to ensure that exposure to radon gas and its radioactive daughter products is as low as possible and does not exceed established safety levels.
- Efficient dust control, because the dust may contain radioactive constituents and emit radon gas.
- Limiting the radiation exposure of workers in mine, mill and tailings areas so that it is as low as possible, and in any event does not exceed the allowable dose limits set by the authorities. In Canada this means that mining in very high-grade ore is undertaken solely by remote control techniques and by fully containing the high-grade ore where practicable.
- The use of radiation detection equipment in all mines and plants, often including personal dose badges.
- Imposition of strict personal hygiene standards for workers handling uranium oxide concentrate.
At any mine, designated employees (those likely to be exposed to radiation or radioactive materials) are monitored for alpha radiation contamination and personal dosimeters are worn to measure exposure to gamma radiation. Routine monitoring of air, dust and surface contamination is undertaken.
Canadian mine and mill facilities are designed to handle safely ore grades of up to 26% U.
If uranium oxide is ingested it has a chemical toxicity similar to that of lead oxide. Similar hygiene precautions to those in a lead smelter are therefore taken when handling it in the drying and packing areas of the mill.
The usual radiation protection procedures are applied at an ISL mine, despite the fact that most of the orebody’s radioactivity remains well underground, and there is hence minimal increase in radon release and no ore dust.
Sustainable development reporting and audit
As well as international quality control standards such as ISO 14001 applying to environmental management at many mines, there is now emerging an industry audit framework in collaboration with consumers of uranium, especially utilities which are sensitive to sustainable development principles, including those of their suppliers. Historically some electric utilities such as Vattenfall and EdF have applied Life Cycle Analysis to include audits of the mines and other fuel cycle facilities supplying them so that they are confident of and can vouch for the standards applying to those activities, both environmentally and socially (especially in relation to indigenous peoples).
The World Nuclear Association has developed a framework for internationally standardized reporting on the sustainable development performance of uranium mining and processing sites. This has been agreed to by the main mining companies and developed in close collaboration with utilities so that they are in a position to report to their stakeholders. WNA is working towards implementation of a common audit program to be used worldwide by utilities and mines. There are moves to involve government regulators in this, since it complements their role, and national mining associations. The data supplied by mines will be subject to a verification process.
Uranium resources and supply
Table 2 shows the current known recoverable resources of uranium by country. Uranium is not a rare element and occurs in potentially recoverable concentrations in many types of geological setting. As with other minerals, investment in geological exploration generally results in increased known resources. Over 2005 and 2006 exploration effort resulted in the world’s known uranium resources increasing by 15% in that two years.
There is therefore no reason to anticipate any shortage of uranium that would prevent conventional nuclear power from playing an expanding role in providing the world’s energy needs for decades or even centuries to come. This does not even take into account improvements in nuclear power technology which could effectively increase the available resource dramatically.
The most common uranium product from mines is U3O8 which contains about 85% uranium.
Uranium resources to $130/kg U by country in 2023 (reasonably assured resources plus inferred resources)
| tonnes U | percentage of world | |
| Australia |
1,671,200 |
28% |
|---|---|---|
| Kazakhstan |
813,900 |
14% |
| Canada |
582,000 |
10% |
| Namibia |
497,900 |
8% |
| Russia | 476,600 | 8% |
| Niger | 336,000 | 6% |
| South Africa | 320,900 | 5% |
| China | 270,500 | 5% |
| Brazil | 167,800 | 3% |
| Mongolia | 144,600 | 2% |
| Ukraine |
106,700 |
2% |
| Botswana |
87,200 |
1% |
| USA |
67,800 |
1% |
| Tanzania | 57,700 | 1% |
| Other |
324,900 |
5% |
| World total |
5,925,700 |
100% |
Identified resources recoverable (reasonably assured resources plus inferred resources), to $130/kg U, 1/1/23, from OECD NEA & IAEA, Uranium 2024: Resources, Production and Demand ('Red Book'). The total recoverable identified resources to $260/kg U is 7.935 million tonnes U.
The current global demand for uranium is about 67,000 tU/yr (tonnes uranium per year). The vast majority is consumed by the power sector with a small amount also being used for medical and research purposes, and some for naval propulsion. At present, about 44% of uranium comes from conventional mines (open pit and underground) about 52% from in situ leach, and 4% is recovered as a by-product from other mineral extraction.
Thus the world's present measured resources of uranium (5.9 Mt) in the cost category above present spot prices and used only in conventional reactors, are enough to last about 90 years. This represents a higher level of assured resources than is normal for most minerals. Further exploration and higher prices will certainly, on the basis of present geological knowledge, yield further resources as present ones are used up.
In the third uranium exploration cycle from 2003 to the end of 2009 about $5.75 billion was spent on uranium exploration and deposit delineation in over 600 projects. In this period over 400 new junior companies were formed or changed their orientation to raise over $2 billion for uranium exploration. About 60% of this was spent on better defining and quantifying previously-known deposits. All this was in response to increased uranium price in the market. However, the market then went into a downward correction, accentuated by the March 2011 accident at Japan's Fukushima Daiichi nuclear plant.
Uranium prices fell by more than 70% following the Fukushima accident to levels around $20/lb in 2016-17. Predictably, as supportive term contracts expired in the late 2010s, many uranium producers – large and small and across multiple regions – elected to suspend or curtail operations and meet supply commitments through existing inventory and purchases. Production challenges continued during the Covid-19 pandemic including slower wellfield development at ISR operations, supply chain disruptions, restrictions and delays on transport and regulatory reviews. Prospects for uranium production changed rapidly in the post-pandemic years with energy policy in many countries favouring nuclear power, increased investor interest and direct purchases of uranium inventories, and the Russia-Ukraine conflict creating uncertainty to uranium supply access under resulting trade restrictions. Current uranium production continues to respond to more favourable market signals with restarts ongoing and more planned.
Secondary sources of uranium
A significant secondary supply of uranium was provided by the decommissioning of nuclear warheads by the USA and Russia under the 'Megatons to Megawatts' programme. Other sources of uranium include government and utility stockpiles and a very large amount of depleted uranium left over from historic enrichment, which can be re-enriched with more efficient processes. A little comes from recycled uranium from reprocessing used fuel.
For detailed information about the main uranium mining countries, see dedicated country profiles.
Economics of uranium mining
The economics of mining uranium involves consideration of several aspects, starting with the country where an orebody is located, then the grade and nature of the ore, its depth, and also infrastructure issues.
Countries have different degrees of sovereign risk affecting their attractiveness for mining investment, different royalty and tax regimes, and different availability of skilled workers. These factors will already have influenced the mineral exploration which has led to the identification of an orebody before any question of mining has arisen.
The quantity and nature of the ore is fundamental. The quantity, geological character and grade, along with its hardness and depth, determine what sort of capital investment is required. The mineral characteristics of the ore determine what sort of processing is required, and affect the cost of both capital and operation.
Infrastructure issues include engineering and workforce. A mine at a remote site costs more. Breaking down this set of considerations into conventional categories:
- Capital costs include the cost of site preparation, construction, manufacture of plant, commissioning and financing both mine and mill. Building a large-scale mine takes many workers, large amounts of steel and concrete, thousands of components, and systems to provide electricity, ventilation (if underground), information, control and communication. Exploration expenditure up to committing to a mine project may or may not be capitalized. Capital costs may be reported with the financing costs included or excluded. If financing costs are included then the capital costs change materially in proportion to construction time of the project and with the interest rate and/or mode of financing employed. Both upfront capital expenditure and some ongoing capital expenditure to sustain the operation are involved in the long term.
- Operating costs include the costs of removing the ore from the ground, concentrating it for sale, the reagents, energy, labour, environmental management, administration, freight, marketing, and a provision for funding the eventual decommissioning and disposal of wastes. Royalty payments to the owner of the minerals (usually the state) are normally included. Normally these costs are expressed relative to a unit of output (for example, US dollars per pound of U3O8, $ per kgU). The ongoing costs of production, both variable and fixed, but excluding depreciation, are sometimes reported as the “cash cost” of an operation per unit of output.
- Indirect costs include depreciation and amortization of the assets, interest on loans, extraordinary costs and possibly the sustaining costs of related exploration and mine development if these are not capitalized. Indirect costs may be hard to quantify for reporting, since they can include non-cash items and servicing capital over the life-of-mine. While the cash cost of production is a short-term metric, including the indirect costs gives a life-of-mine perspective.
Taking all these into account, several escalating categories of cost reporting metrics can be distinguished:
C1: Cash operating cost, as above
C2: Total production cost, including depreciation
AISC: All-in sustaining cost, including development and related costs to sustain future production
C3: Fully allocated cost, including all costs of the business.
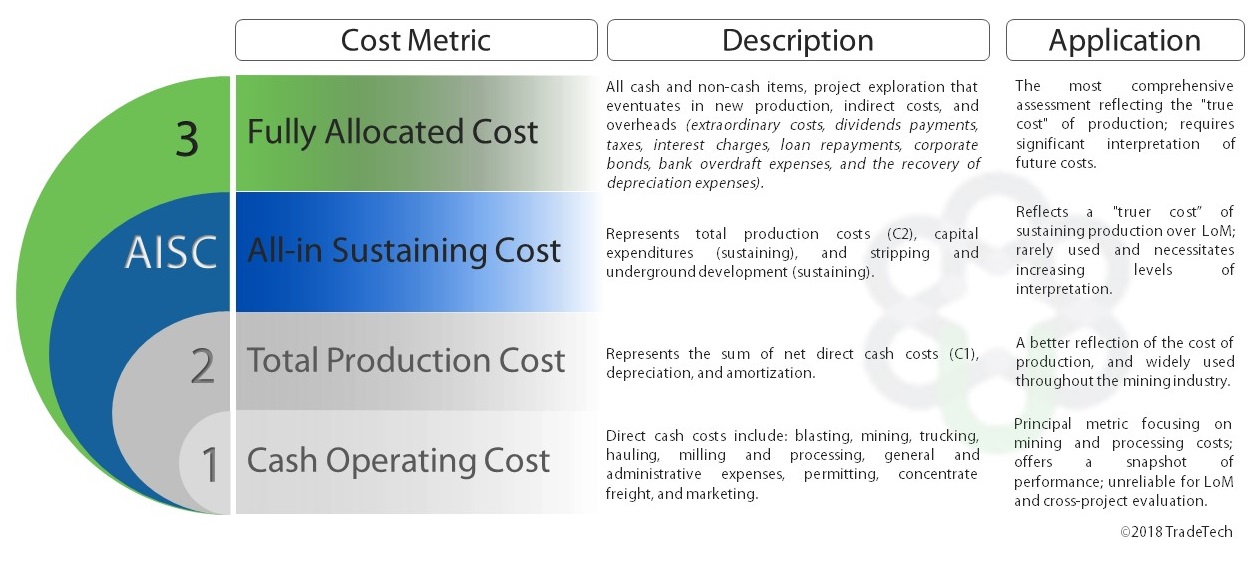
Source: TradeTech
TradeTech has compared the cost profiles of a number of uranium mines for reporting their C3 operating costs per unit of output. In particular the comparison between open pit and in situ leach (ISL) operations is interesting. It shows that for ISL mines, almost half the C3 cost is capital in setting up the operation, another quarter is sustaining it, and only just over one quarter is the basic C1 cash cost.* With open pit mines the basic C1 operating cost is a much higher proportion – about 70% – and only 20% is capital in setting up the operation, plus a bit more in sustaining it.
* The 2017 World Nuclear Association Nuclear Fuel Report noted that ISL mines “require continuing investment in wellfield development in order to maintain capacity.“
This comparison underlines the need to be aware of what is included in any quoted production cost figures, though comparing C1 costs for very similar projects may be useful. Ultimately, the economic viability of any mine depends on the C3 figures, but the difficulty in quantifying this at any point during operation means that a more realistic comparative metric is AISC.
Issues related to uranium mining
Safeguards to prevent military use
Among uranium-exporting countries, Australia and Canada have some of the strictest conditions relating to the use of its uranium. These safeguards (inspections and accounting procedures) ensure that exported uranium is used for peaceful purposes only and is not diverted for military purposes or used in a way which adds to the proliferation of nuclear weapons.
Bilateral agreements to this effect between the Australian and Canadian governments and each country wishing to import their uranium are therefore necessary before sales contracts can be completed. Such agreements are in addition to the application of International Atomic Energy Agency (IAEA) safeguards administered under the Nuclear Non-Proliferation Treaty. The further transfer of nuclear material is only permitted to countries which have bilateral safeguards agreements with Australia or Canada.
Australia, Canada and Kazakhstan are now the world's major producers and exporters of uranium. In addition to providing further diversification and strength to their domestic economies, it gives all three countries a voice in the framing of international nuclear policies and safeguards. It also reduces the need for buyers to seek uranium from countries with less effective safeguards.
Some uranium basics
The atomic number of uranium is 92, meaning that it has 92 protons and occupies place number 92 in the periodic table. It occurs naturally in six isotopes, U-233 to U-238 and therefore contains between 141 and 146 neutrons. The most common isotope is U-238 with a relative abundance of 99.3%. The second most common is U-235 with a relative abundance of 0.7% and the rest occur in trace amounts. All isotopes of uranium are radioactive and over time they decay to other lighter elements. However the rate of decay is slow; the radioactive half-life of U-238 is 4.47 billion years, meaning that it takes this much time for half of any given sample of U-238 to break down. The half-life of U-235 is 704 million years which means that most of the Earth's original U-235 has already decayed away.
A further property of U-235 is that it is fissile and so neutrons emitted during fission can cause other U-235 nuclei to fission also, releasing a lot of energy. This reaction is the basis of operation for the world’s current nuclear power stations and is the major reason why uranium is a valuable mineral resource.